OVERVIEW
A double-blind, placebo-controlled clinical trial of Qualia Senolytic showed a statistically significant reduction in total scores of issues related to joint health and performance, which includes Joint Discomfort, Physical Function, and Joint Stiffness. The most promising result was an average 68% reduction in joint discomfort. Secondary endpoints were also studied using the Rand SF-36 Quality of Life Metrics which includes Physical Functioning, Role Functioning Physical, Role Functioning Emotional, Energy/Fatigue, Emotional Well-Being, Social Functioning, Discomfort and General Health. All metrics showed substantial improvement from baseline testing, with several improving to a greater degree than was observed in the placebo control group. Though these secondary endpoints didn’t quite meet the standard for statistical significance, multiple endpoints were very close and are highly encouraging indicators of additional health benefits beyond the statistically significant joint-related benefits.
HOW THE STUDY WORKED
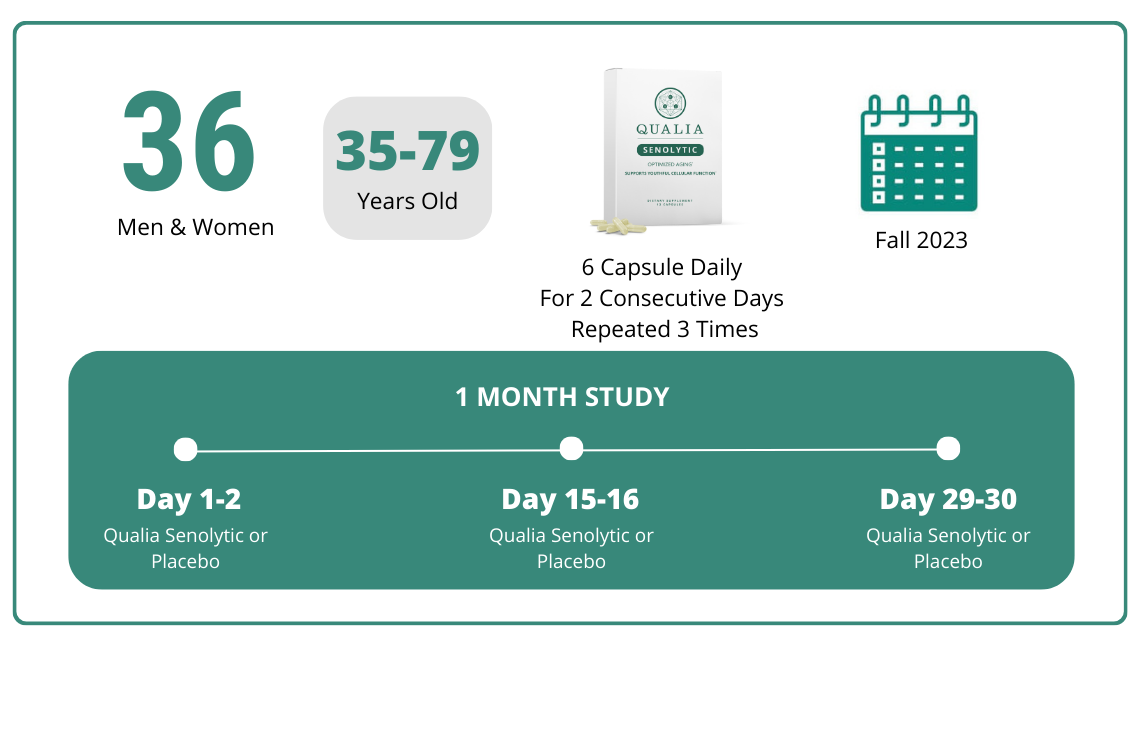
Thirty-six adults participated in the study and had their baseline and final data statistically analyzed. Participants ranged in age from 35 to 79 years old, and were a mix of males and females. All participants had at least a moderate degree of difficulty in comfortably performing a variety of activities of daily living.
The study was double-blinded, which means that neither the researchers conducting the study nor the participants knew whether they’d received Qualia Senolytic or placebo capsules. Participants were randomly assigned to be in the Qualia Senolytic or placebo group.
All participants were instructed to take six capsules of the product they’d received on two consecutive days anytime in the morning, with or without food. After these two days, there was a twelve day period with no supplementation, followed by the start of the next two-day supplementation cycle. Participants completed three of the two-day supplementation cycles during the study, which means that they took either Qualia Senolytic or a placebo for a total of six days over about a one month time period.
The primary study endpoint was the total score on a questionnaire that asked about various aspects of joint health and performance. Secondary endpoints were subscales from this questionnaire that related to joint comfort, ability to comfortably perform routine activities of daily living, and experiences related to occasional stiffness.
A separate questionnaire—RAND 36-Item Short Form Survey Instrument (SF-36)—was also used as a secondary endpoint. The SF-36 is one of the most widely used tools to assess health status and health-related quality of life.
Participants completed both sets of questionnaires—joint health and performance as well as the SF-36—prior to taking the capsules they’d received and again after the third two-day supplementation cycle. Initial and final values were evaluated for statistical significance within groups and between groups.
RESULT HIGHLIGHTS
Support for Joint Health and Performance*
Evaluating joint health and performance was a primary goal of the study because the cells that joints rely on for healthy structure and function—fibroblasts, endothelial cells, tissue-resident macrophages, and chondrocytes—are among the most susceptible to cellular senescence. The general idea is that, by supporting the natural processes that remove senescent joint cells, it allows the stressed, older cells to be replaced with younger, healthier cells. This is then experienced as better joint performance.*
Qualia Senolytic improved the total score, and the discomfort, physical function, and stiffness subscores compared to baseline (p<0.001).* The total score reflects participants’ self-rated experiences of joint health and performance. It is the sum of discomfort, physical function, and stiffness subscores. The physical function subscore captures routine activities of daily living—bending over, getting up from sitting or into and out of things, household tasks, walking, etc. These types of activities can become more difficult to perform comfortably as we age and are at the center of healthy physical function. It’s also common to experience some degree of loss of flexibility as we get older. We may feel a bit stiffer in the morning, at other times during the day, or when we are performing routine activities. This occasional stiffness that can accompany aging is captured in the stiffness subscore. The image below shows initial scores, final scores, and percent decreases for the joint health and performance scores and subscores in the Qualia Senolytic and placebo groups.
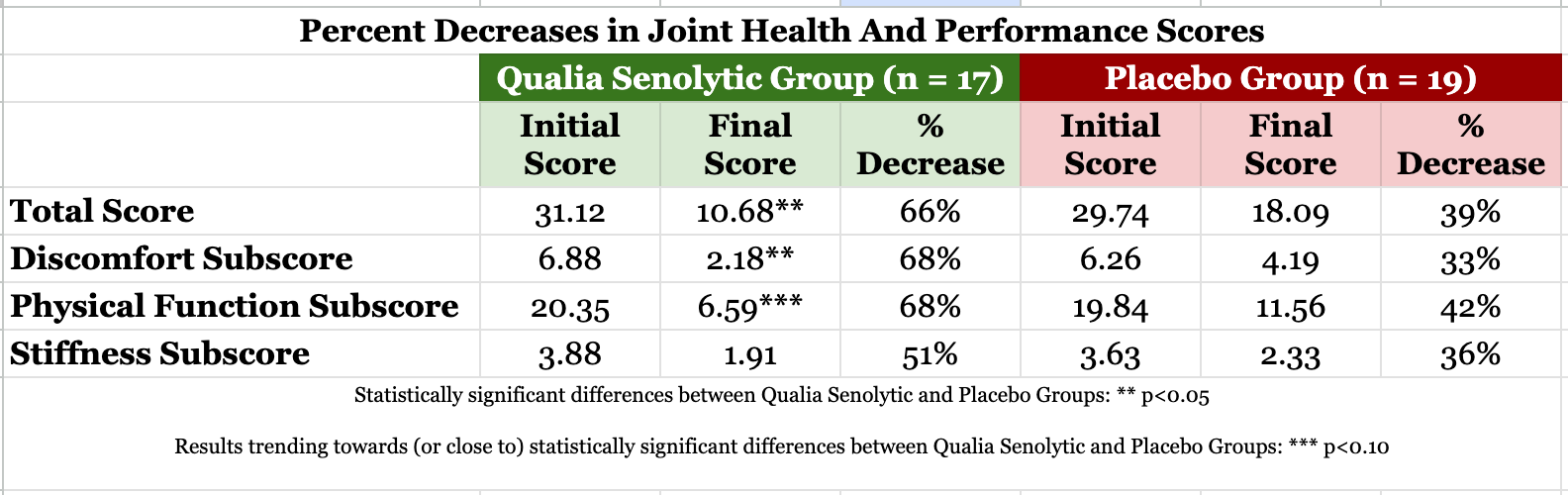
Note: Because of the design of the joint health and performance questionnaire, decreases in scores indicate improvements, with greater decreases meaning more improvement.*
Despite the large placebo response, Qualia Senolytic significantly outperformed the placebo. The total score for joint health and performance improved significantly in the group receiving Qualia Senolytic compared to the placebo group (p=0.05). The improvement in the discomfort subscore was also statistically significant compared to the placebo group (p=0.02). The physical function subscore trended toward statistically significant improvement compared to placebo (p=0.10). Improvement in stiffness subscore was greater in the Qualia Senolytic group than placebo group, but this difference didn’t meet the standard for statistical significance (p = 0.14), though is still encouraging.*
Support for Health Status and Health-Related Quality of Life*
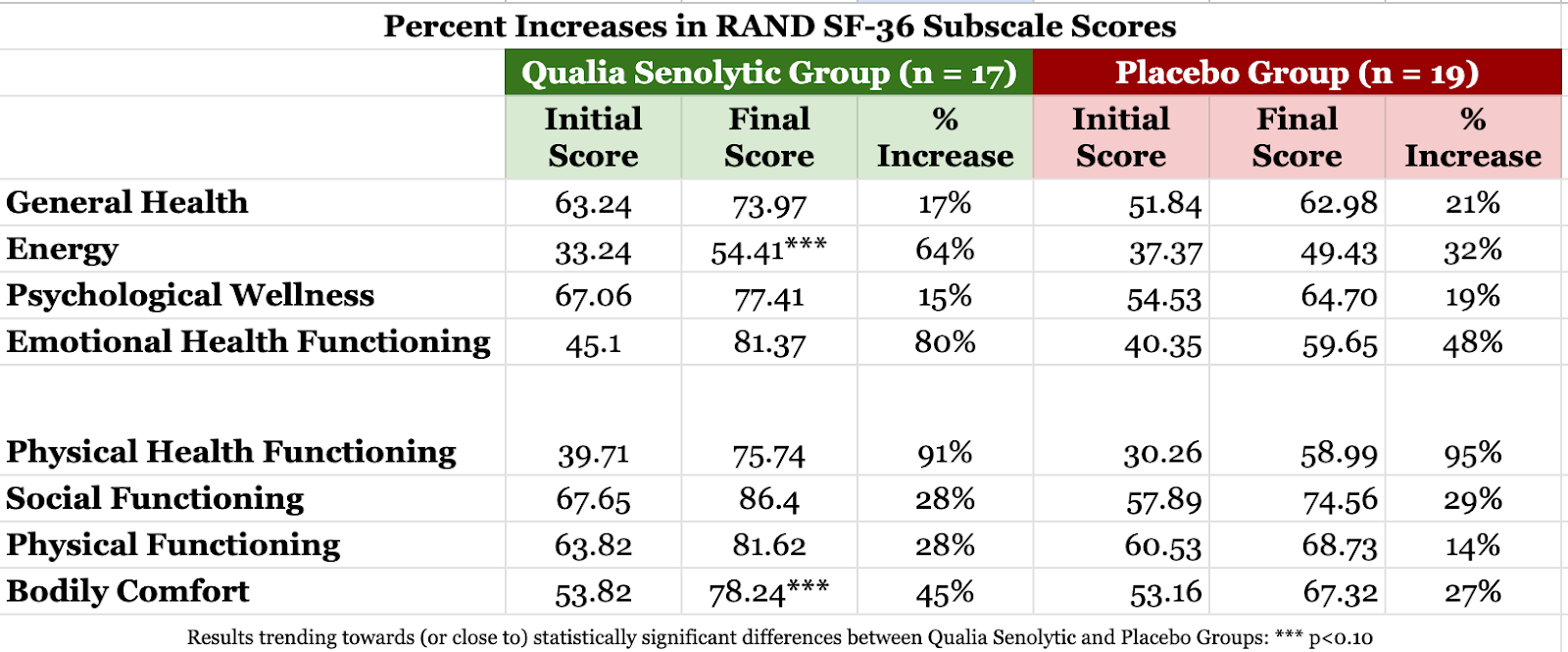
The SF-36 scores eight health concepts: (1) General Health, (2) Energy, (3) Psychological Wellness (4) Emotional Health Functioning (i.e., to what degree emotional health influences time spent and ability to accomplish work or daily activities), (5) Physical Health Functioning (i.e., to what degree physical health influences time spent and ability to accomplish work or daily activities), (6) Social Functioning (i.e., ability to engage in normal social activities), (7) Physical Functioning (i.e., ability to perform physical activities), and (8) Bodily Comfort (i.e. occasional pain and how much it interferes with work). Higher scores indicate better performance in these areas. All eight of the health concepts assessed by the RAND SF-36 improved significantly from baseline after taking Qualia Senolytic (p≤0.01).*
Greatest improvements from baseline levels for the group of participants taking Qualia Senolytic occurred with: (1) Physical Health Functioning (91% improvement), (2) Emotional Health Functioning (80% improvement), (3) Energy (64% improvement), and (4) Bodily Comfort (45% improvement).*
The image below shows initial scores, final scores, and percent decreases for SF-36 health concepts in the Qualia Senolytic and placebo groups. The energy (p=0.09) and bodily comfort (p=0.07) health concepts trended toward statistically significant improvement for Qualia Senolytic compared to placebo.*
*These statements have not been evaluated by the Food and Drug Administration. The products and information on this website are not intended to diagnose, treat, cure or prevent any disease. The information on this site is for educational purposes only and should not be considered medical advice. Please speak with an appropriate healthcare professional when evaluating any wellness related therapy. Please read the full medical disclaimer before taking any of the products offered on this site.

Carol Church