What is Akkermansia Good For?
Akkermansia muciniphila (A. muciniphila) is a bacterial species that inhabits the human gut. A. muciniphila lives in the mucus layer that covers the intestinal wall, where it specializes in degrading mucus and using it as its energy source, helping to stimulate mucus turnover, which is essential for gut health.
A. muciniphila has key roles in maintaining the integrity of the intestinal barrier, supporting a healthy gut microbiota composition, modulating immune responses, influencing metabolic pathways, and promoting healthy aging. In virtue of its many benefits to human physiology and its roles in supporting human health and the gut microbiota, A. muciniphila is regarded as a promising next-generation probiotic.
Akkermansia has key roles in maintaining the integrity of the intestinal barrier, supporting a healthy gut microbiota composition, modulating immune responses, influencing metabolic pathways, and promoting healthy aging.
What is Akkermansia?
Akkermansia is a genus in the phylum Verrucomicrobiota. Because Akkermansia muciniphila is the only member of the genus identified in the human gut, when we use the term Akkermansia in the context of the human gut microbiota, we refer specifically to Akkermansia muciniphila.
A. muciniphila was identified in 2004 by Dutch researchers [1] who named it Akkermansia as a tribute to the Dutch microbiologist Antoon Akkermans, recognized for his contribution to microbial ecology, and muciniphila (meaning mucin-loving) based on its ability to break down a type of sugary protein called mucin in the gut.
A. muciniphila represents approximately 1–3% of the total microbial population of the colon of healthy people [2]. It is one of the top 20 most abundant species in the human gut [3] and it is detectable in the gut microbiota in all stages of life [4]. A. muciniphila is present in breast milk, through which it can be transferred to infants [5]. Within the first year of life, A. muciniphila stably colonizes the gut and its abundance reaches the same level as that of healthy adults; it then gradually decreases in the elderly [2,4,6].
A. muciniphila is also found in most species of vertebrates that have been sampled so far [7], suggesting we may have a long evolutionary link with A. muciniphila. Perhaps because of this co-evolution, A. muciniphila and humans appear to live in a mutualistic symbiosis in which both species benefit from their association: A. muciniphila gets its energy from molecules produced by our body, while we get physiological and health benefits from its activity.
Akkermansia Lives In the Mucus Layer of the Gut
The gastrointestinal tract is covered by a layer of mucus produced by specific epithelial cells called Goblet cells. Mucus acts as a lubricant and a protective barrier against harmful microbes and mechanical damage. The structure of the mucus layer varies throughout the gut. In the small intestine, mucus forms only a single, loosely attached layer. In the colon, mucus forms two layers: an inner layer that, in normal conditions, is devoid of bacteria and acts as an additional physical barrier against microbial invasion, and an outer mucus layer where only specific bacteria can survive [8–10]. The outer mucus layer of the colon is the ecological niche where A. muciniphila primarily lives. In the small intestine, only small amounts of A. muciniphila are found [11].
A. muciniphila’s presence in the mucus layer in close proximity to the cells of the intestinal wall and its capacity to degrade mucin are distinctive features that underlie its far-reaching influence and make it a keystone species of the gut microbiota [12]. Keystone species are organisms that have considerable influence on their ecosystem irrespective of their abundance [13,14]. Because of their unique and crucial roles, keystone species are especially important for the stability of their ecosystem and their absence can cause a dramatic shift in their environment. This is the case for Akkermansia and the gut ecosystem.
Keystone species, like akkermansia, are especially important for the stability of their ecosystem and their absence can cause a dramatic shift in their environment.
How Does Akkermansia Work?
Mucin Degradation
Mucus is composed primarily of glycoproteins (i.e., proteins bound to carbohydrate molecules) called mucins (and collectively called mucin). One of the defining features of A. muciniphila is its ability to degrade mucin through specific enzymes it produces and use it as its sole source of carbon, nitrogen, and energy [1].
Mucus breakdown by A. muciniphila generates short-chain fatty acids (SCFAs) as byproducts, namely acetate, propionate, and the butyrate precursor succinate [1]. SCFAs, which are also produced by other microbial species in the metabolism of non-digestible complex sugars (e.g., starch and fiber), have many important roles in gut homeostasis. SCFAs can be used as nutrients by other microbial species in the gut. SCFAs can also be absorbed into the bloodstream and distributed to other tissues and organs, including the brain, where they can modulate physiological responses [15].
Mucin breakdown by A. muciniphila also produces sugars, amino acids, sulfate, and other molecules that can be used as energy sources by other gut microbes and promote their growth [7,16,17]. Other gut microbes can also use these molecules to produce additional SCFAs, namely butyrate [18].
In clinical studies, higher akkermamsia abundance was found to be correlated with higher microbiome richness.
By converting mucin into byproducts that benefit other microbes, A. muciniphila may stimulate a balanced and healthy microbial composition in the gut [2]. Gut microbiome researchers call this sharing of metabolites between different species “cross-feeding”: it is essential for maintaining a thriving community of gut bacteria that is stable, resilient, and able to resist unwanted organisms from colonizing [19]. In clinical studies, higher A. muciniphila abundance was found to be correlated with higher microbiome richness [20,21]. These actions may support beneficial microbiota-host interactions and health-promoting physiological responses.
Akkermansia Supports Gut Barrier Function
If in the future someone should ask you why Akkermansia is important, one of the first things that should come to mind is that it reinforces the gut barrier. The intestinal barrier is crucial for isolating our body from gut microbes. A physical barrier is created by the epithelial cells that line the gut, the molecules on their surface, and the mucus they produce, while a chemical barrier is created by immune signaling molecules, antibodies, and antimicrobial substances produced by epithelial and immune cells. But the gut barrier does more than isolate our body from microbes: it is a dynamic interface where beneficial interactions between host and microbes also take place.
The mucus layer plays an important part in maintaining gut barrier function. Mucus must be continuously refreshed to create a healthy environment for the epithelial cells of the intestinal wall. A. muciniphila degrades mucin and breaks down the upper loose layer of mucus. While doing so, it also stimulates new mucus production and mucus thickening [22–24].
These housekeeping actions of A. muciniphila are essential for balanced intestinal self‐renewal and gut health. By supporting mucus turnover, A. muciniphila helps to maintain a healthy mucus layer that protects the integrity of intestinal epithelial cells, reinforces the intestinal barrier, and reduces gut permeability, which provides resistance to invasion by harmful microbes and their metabolites [6,22,25]. Additionally, new mucus production stimulates the growth of beneficial bacteria [3].
Animal studies have shown that supplementation with A. muciniphila supports gut barrier function by increasing the number of mucin-producing goblet cells, supporting mucus layer thickness, and promoting the expression of tight-junction proteins that reinforce the physical barrier in the gut [22,26–28].
A low abundance of akkermansia may result in the thinning of the mucosa and a weakening of the intestinal barrier function, leading to what is commonly known as a leaky gut.
On the other hand, a low abundance of A. muciniphila may result in the thinning of the mucosa and a weakening of the intestinal barrier function, leading to what is commonly known as a leaky gut: it makes it easier for harmful microbes and toxins to invade our body [29].
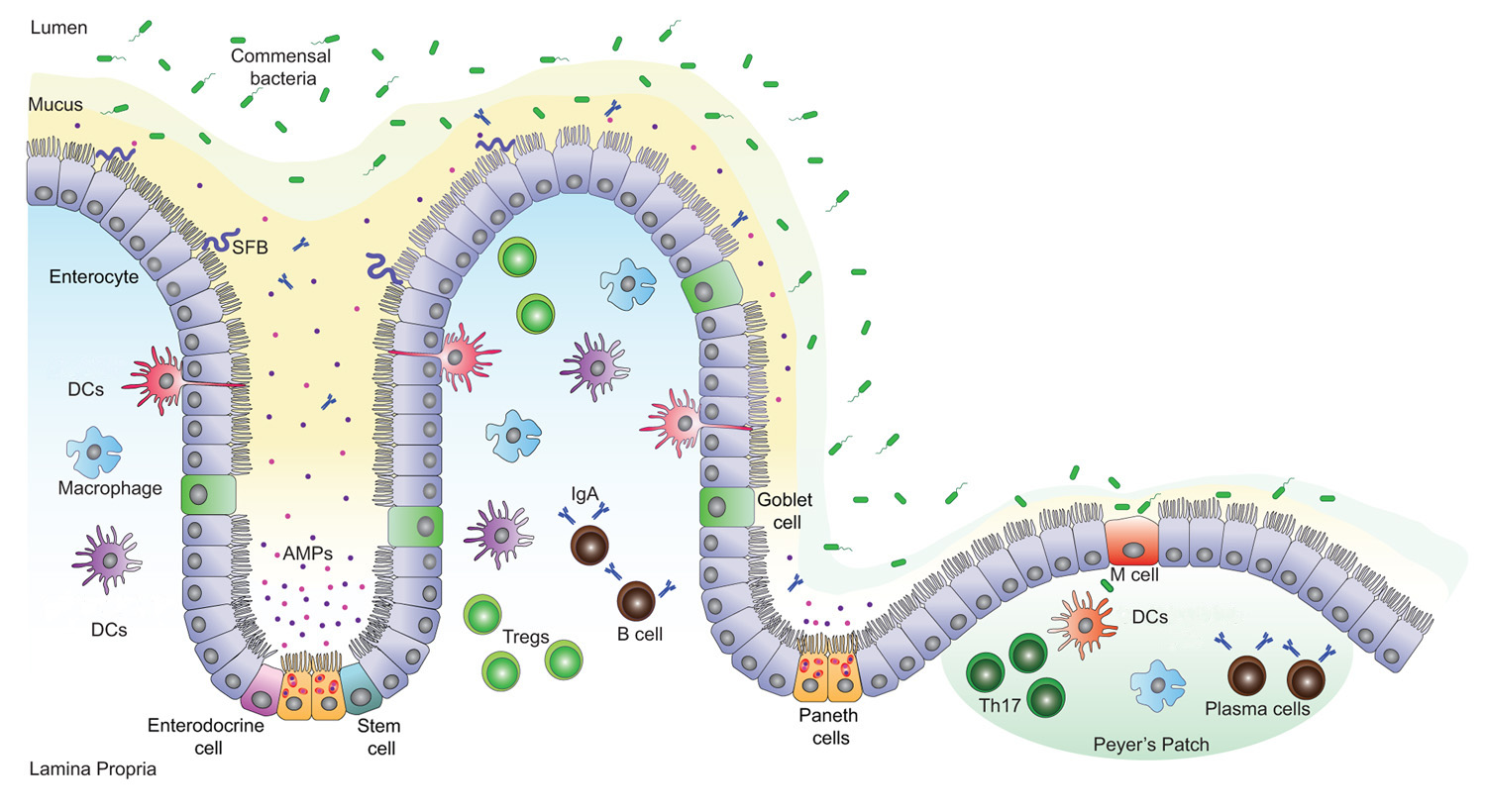
Figure 1. Intestinal epithelial barrier and immune cells (Adapted from Muniz et al, 2012; 10.3389/fimmu.2012.00310. License: CC BY 4.0)
Akkermansia is Critical in Immune Function
As a keystone species in the gut, and the dominant bacteria in the mucus layer, Akkermansia plays a critical role in gut mucosal immunity, helping to keep the immune system in balance. As home to around 38 trillion microbes (roughly as many as cells in the human body) [30], the gut plays an important part in our immune defense system. The immune system helps to create a chemical barrier in the gut by producing and stimulating the production by other cells of immune mediators, antibodies, and antimicrobial substances.
The immune system and the gut microbiota are in permanent crosstalk and mutual influence, working together to devise protective responses against harmful microbes and promote tolerance to friendly microbes and their products [31]. Microbes that live on the surface of the intestinal mucosa are particularly important for this crosstalk with host immunity [32].
The immune system and the gut microbiota are in permanent crosstalk and mutual influence, working together to devise protective responses against harmful microbes and promote tolerance to friendly microbes and their products.
Accordingly, A. muciniphila plays an important part in supporting mucosal immune responses and promoting the production of antimicrobial peptides by secretory cells in the intestinal wall, thereby helping to reinforce the immune barrier of the gut [6,33]. A. muciniphila may influence intestinal immune function by modulating immune cell function, inducing the production of immune signaling molecules, and promoting the expression of receptors involved in the recognition of microbial molecules (toll-like receptors) [34–36].
A. muciniphila can actively communicate with the immune system through different mechanisms. SCFAs produced by A. muciniphila are one of the most relevant. Activation of SCFA receptors in host cells can trigger signaling pathways that influence immune cell function and signaling, support immune responses, and help promote the integrity of the epithelial barrier [25,33,37–39].
The effects of A. muciniphila can also be mediated by molecules found at its surface (e.g., Amuc_1100). These molecules can activate receptors that recognize microbial structures expressed in immune and epithelial cells of the intestinal wall and that influence immune responses in the host [34,40,41]. A. muciniphila may also influence host physiological responses through secreted proteins and extracellular vesicles [42,43].
Akkermansia For Metabolic Function
One of Akkermansia’s most studied actions has been how it interacts with human metabolic health, including gaining or losing weight, blood sugar regulation, and liver health.
As mentioned, A. muciniphila produces SCFAs. Microbial SCFAs influence several aspects of human metabolism, both by serving as an energy source and by influencing gene expression and signaling pathways [15]. In the gut, SCFAs can serve as fuel for epithelial cells that line the intestinal wall and modulate their metabolism and secretions [44]. SCFAs can also be absorbed into the bloodstream and transported to other tissues [15]. The SCFA propionate is used by the liver to produce glucose, while acetate is transported in the blood to other tissues where it can be used as a substrate for energy metabolism [45–47].
SCFAs are also signaling molecules. SCFAs bind to specific receptors expressed by endocrine cells of the intestinal wall and influence nutrient-sensing and gut hormone secretion [45,48,49]. SCFAs also act on liver and muscle cells to regulate glucose utilization and energy expenditure, and on adipose cells to regulate fat storage and energy homeostasis [49–51].
SCFA receptors are also expressed in neurons of the enteric nervous system (the nervous system of the gut) and of the Vagus nerve [50,51]. Through these receptors, SCFAs can influence the gut-brain axis and regulate appetite, food intake, metabolism, and energy homeostasis [52–54].
In studies akkermansia influenced energy metabolism, improved metabolic markers, body weight, and body composition, and showed a causal relationship with improved metabolic health.
A. muciniphila supplementation has shown beneficial actions in the prevention and amelioration of metabolic dysfunction [55]. In mouse models, A. muciniphila influenced energy metabolism, improved metabolic markers, body weight, and body composition, and showed a causal relationship with improved metabolic health [22,41,56]. In humans, supplementation with A. muciniphila in overweight human volunteers improved several metabolic parameters [57] and a better metabolic profile was observed in individuals harboring more A. muciniphila, as well as better metabolic responses to calorie restriction [21].
How Gut Dysbiosis Impacts Aging
In 2023, three new areas were added to the Hallmarks of Aging [58]. One of the new additions was dysbiosis, which means that there is an imbalance in the gut microbial community. One of the organisms that’s being found to play an important role in the health of the gut microbiome with aging is Akkermansia.
The composition of the gut microbiota changes gradually with age [59] and these alterations are believed to be associated with age-related health decline in older adults [60]. Studies have found that A. muciniphila is more abundant in the gut microbiota of healthy older adults or long-lived individuals (centenarians) compared to non-healthy older adults [61–63]. Also, a study with centenarians observed a significant reduction in A. muciniphila as their health gradually deteriorated [64]. These findings suggest that changes in A. muciniphila abundance may also contribute to aging and age-related health decline.
Preclinical studies have supported the role of A. muciniphila in promoting healthy aging. In studies with aged mice, supplementation with A. muciniphila improved age-related changes in immune cell function, immune signaling, systemic immunity, redox status, metabolic markers, mucus layer thickness, gut barrier function, and gut homeostasis; it also restored cognitive function and muscle atrophy [24,65–67].
These studies suggest that A. muciniphila may be associated with a prolonged healthspan and healthy aging, possibly by helping to mitigate age-related changes in metabolic and immune function, intestinal barrier function, and the gut microbiota that can accelerate the aging process [68].
A. muciniphila may do so by supporting healthy SCFA levels in the host, as a reduction in SCFAs is one of the changes observed with aging [69]. SCFA plasma levels are associated with metabolic health [70,71], healthy blood pressure [72], and healthy brain function [73,74], for example.
Disruption of the intestinal barrier is also associated with intestinal, metabolic, and immune dysfunctions that can contribute to age-related health decline. By helping to maintain gut microbial homeostasis and a healthy mucus layer and gut barrier, A. muciniphila may also support healthy aging [22,28,68].
How May Akkermansia Help as a Supplement?
The abundance of A. muciniphila in the human gut correlates with host health. But the abundance of A. muciniphila can be affected by several factors, including age, diet, and metabolic, immune, intestinal, and general health status [16,75].
A reduced abundance of A. muciniphila in the gut microbiota has been linked to intestinal, metabolic, and immune dysfunctions that can contribute to poorer health [75].
A reduced abundance of akkermansia in the gut microbiota has been linked to intestinal, metabolic, and immune dysfunctions that can contribute to poorer health.
Because several conditions affect its abundance, A. muciniphila is regarded as a health marker [11].
A. muciniphila’s roles in supporting gut health, immune defenses, and metabolic function make it a promising probiotic [3]. Clinical studies with A. muciniphila are still limited, but dietary supplementation has been shown to improve metabolic parameters in overweight individuals [57]. Animal studies have supported the value of A. muciniphila as a probiotic for human health, particularly in promoting gut barrier function, immune signaling and defenses, metabolism and energy homeostasis, healthy gut microbiota composition, and healthy aging [11,68].
There are different approaches to promote healthy A. muciniphila levels in the gut. Dietary supplementation is one of the simplest strategies. A. muciniphila can be supplemented in two main forms: live or inactivated by heat treatment. Heat-inactivated A. muciniphila retains some of its properties because proteins at its surface that activate receptors in cells of the intestinal wall have high heat stability.
Both live and heat-inactivated A. muciniphila were well tolerated in a clinical trial [57]. Both forms have been found to support gut barrier function, immune responses, and metabolic function, although there have been conflicting results regarding their relative efficacy [41,55,76]. However, one study showed that only live A. muciniphila was able to support mucus layer thickness [22]. Supplementation with live A. muciniphila has consistently been shown in multiple studies to increase its abundance in the gut [77], allowing it to exert its specialized function of degrading mucin and promoting mucus turnover.
Prebiotics are things we can eat (usually fibers) that are precursors for microbial life. They are substrates that act like food for gut microbiota, providing nourishment for and supporting the growth of specific gut bacteria [78]. Supplementation with prebiotics, such as fructooligosaccharides and inulin, has also been shown to promote A. muciniphila abundance [77,79,80]. In a human clinical study, a resistant starch made from potatoes (SolnulTM) supported an increase in the relative abundance of A. muciniphila [81].
Dietary polyphenols, which are natural antioxidants that may reduce oxidative stress in the gut and support gut health, can also have a prebiotic-like role that may potentially support A. muciniphila abundance [82,83]. Polyphenols found in foods like berries, grapes, tea (green or black), and pomegranate support the growth of A. muciniphila [84].
Pomegranate is a polyphenol-rich food that has been shown to support a healthy gut microbiota [85–87]. In a human study, pomegranate extract increased A. muciniphila abundance but only in urolithin A producers (urolithin A is a metabolite of ellagitannins that are found in high amounts in pomegranate) compared to non-producers [86]. In fact, urolithin A producers had 33-fold higher levels of A. muciniphila at baseline and this difference was increased to 47-fold higher levels of A. muciniphila compared to non-producers. This suggested that A. muciniphila might play an important role in the breakdown of phenolic compounds in the intestine and that different individuals may benefit differently from polyphenol supplementation for promoting A. muciniphila abundance [86].
Because A. muciniphila is a mucin degrader, it is affected by the availability and quality of mucin. Polyphenols may support A. muciniphila growth, at least in part, by enhancing the quality of mucin, fortifying it, so to speak [84]. Ingredients that support mucin production may also support the growth of A. muciniphila. The amino sugar N-acetylglucosamine is a precursor for some of the glycans found in mucin that can support intestinal mucin production and A. muciniphila growth [88]. This ability to support A. muciniphila abundance was one of the reasons why we included N-acetylglucosamine in Qualia Synbiotic.
Qualia Synbiotic is a combination of prebiotics, probiotics, postbiotics, fermented foods, herbs, and digestive enzymes that work complementarily to support digestive health, gastrointestinal performance, the gut microbiota, the gut-immune axis, and the gut-brain axis.* We developed Qualia Synbiotic to support gut health, gastrointestinal performance, and the gut-brain axis. In addition to N-acetylglucosamine, Qualia Synbiotic contains other ingredients that may potentially support A. muciniphila growth. SolnulTM, a resistant starch made from potatoes, supported an increase in the relative abundance of A. muciniphila in a human clinical study [81]. Berriotics™, a postbiotic produced by fermenting ten berries, is rich in polyphenols that support a healthy gut microbiota [89]. Because tea has been shown to promote the growth of A. muciniphila [90], InstaKombu™, a combination of fermented kombucha with apple cider vinegar and fiber, may also contribute to supporting A. muciniphila abundance.
And, get excited. COMING SUMMER 2024: Qualia Akkermansia.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
References
[1]M. Derrien, E.E. Vaughan, C.M. Plugge, W.M. de Vos, Int. J. Syst. Evol. Microbiol. 54 (2004) 1469–1476.
[2]M. Derrien, M.C. Collado, K. Ben-Amor, S. Salminen, W.M. de Vos, Appl. Environ. Microbiol. 74 (2008) 1646–1648.
[3]T. Zhang, Q. Li, L. Cheng, H. Buch, F. Zhang, Microb. Biotechnol. 12 (2019) 1109–1125.
[4]M.C. Collado, M. Derrien, E. Isolauri, W.M. de Vos, S. Salminen, Appl. Environ. Microbiol. 73 (2007) 7767–7770.
[5]M.C. Collado, K. Laitinen, S. Salminen, E. Isolauri, Pediatr. Res. 72 (2012) 77–85.
[6]M. Derrien, C. Belzer, W.M. de Vos, Microb. Pathog. 106 (2017) 171–181.
[7]C. Belzer, W.M. de Vos, ISME J. 6 (2012) 1449–1458.
[8]M.E.V. Johansson, J.M.H. Larsson, G.C. Hansson, Proc. Natl. Acad. Sci. U. S. A. 108 Suppl 1 (2011) 4659–4665.
[9]M.E.V. Johansson, M. Phillipson, J. Petersson, A. Velcich, L. Holm, G.C. Hansson, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 15064–15069.|
[10]M. Herath, S. Hosie, J.C. Bornstein, A.E. Franks, E.L. Hill-Yardin, Front. Cell. Infect. Microbiol. 10 (2020) 248.
[11]R. Ghotaslou, E. Nabizadeh, M.Y. Memar, W.M.H. Law, M.A. Ozma, M. Abdi, M. Yekani, H. Kadkhoda, R. Hosseinpour, S. Bafadam, A. Ghotaslou, H.E. Leylabadlo, J. Nezhadi, Microbiol. Res. 266 (2023) 127245.
[12]H. Tudela, S.P. Claus, M. Saleh, Front Cell Dev Biol 9 (2021) 719072.
[13]R.T. Paine, Conserv. Biol. 9 (1995) 962–964.
[14]S. Banerjee, K. Schlaeppi, M.G.A. van der Heijden, Nat. Rev. Microbiol. 16 (2018) 567–576.
|[15]B. van der Hee, J.M. Wells, Trends Microbiol. 29 (2021) 700–712.
16]A. Pellegrino, G. Coppola, F. Santopaolo, A. Gasbarrini, F.R. Ponziani, Nutrients 15 (2023).
[17]N. Ottman, M. Davids, M. Suarez-Diez, S. Boeren, P.J. Schaap, V.A.P. Martins Dos Santos, H. Smidt, C. Belzer, W.M. de Vos, Appl. Environ. Microbiol. 83 (2017).
[18]C. Belzer, L.W. Chia, S. Aalvink, B. Chamlagain, V. Piironen, J. Knol, W.M. de Vos, MBio 8 (2017).
[19]E.J. Culp, A.L. Goodman, Cell Host Microbe 31 (2023) 485–499.
[20]E. Le Chatelier, T. Nielsen, J. Qin, E. Prifti, F. Hildebrand, G. Falony, M. Almeida, M. Arumugam, J.-M. Batto, S. Kennedy, P. Leonard, J. Li, K. Burgdorf, N. Grarup, T. Jørgensen, I. Brandslund, H.B. Nielsen, A.S. Juncker, M. Bertalan, F. Levenez, N. Pons, S. Rasmussen, S. Sunagawa, J. Tap, S. Tims, E.G. Zoetendal, S. Brunak, K. Clément, J. Doré, M. Kleerebezem, K. Kristiansen, P. Renault, T. Sicheritz-Ponten, W.M. de Vos, J.-D. Zucker, J. Raes, T. Hansen, MetaHIT consortium, P. Bork, J. Wang, S.D. Ehrlich, O. Pedersen, Nature 500 (2013) 541–546.
[21]M.C. Dao, A. Everard, J. Aron-Wisnewsky, N. Sokolovska, E. Prifti, E.O. Verger, B.D. Kayser, F. Levenez, J. Chilloux, L. Hoyles, MICRO-Obes Consortium, M.-E. Dumas, S.W. Rizkalla, J. Doré, P.D. Cani, K. Clément, Gut 65 (2016) 426–436.
[22]A. Everard, C. Belzer, L. Geurts, J.P. Ouwerkerk, C. Druart, L.B. Bindels, Y. Guiot, M. Derrien, G.G. Muccioli, N.M. Delzenne, W.M. de Vos, P.D. Cani, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 9066–9071.
[23]N.-R. Shin, J.-C. Lee, H.-Y. Lee, M.-S. Kim, T.W. Whon, M.-S. Lee, J.-W. Bae, Gut 63 (2014) 727–735.
[24]B. van der Lugt, A.A. van Beek, S. Aalvink, B. Meijer, B. Sovran, W.P. Vermeij, R.M.C. Brandt, W.M. de Vos, H.F.J. Savelkoul, W.T. Steegenga, C. Belzer, Immun. Ageing 16 (2019) 6.
[25]J. Reunanen, V. Kainulainen, L. Huuskonen, N. Ottman, C. Belzer, H. Huhtinen, W.M. de Vos, R. Satokari, Appl. Environ. Microbiol. 81 (2015) 3655–3662.
[26]C. Chelakkot, Y. Choi, D.-K. Kim, H.T. Park, J. Ghim, Y. Kwon, J. Jeon, M.-S. Kim, Y.-K. Jee, Y.S. Gho, H.-S. Park, Y.-K. Kim, S.H. Ryu, Exp. Mol. Med. 50 (2018) e450.
[27]C. Grander, T.E. Adolph, V. Wieser, P. Lowe, L. Wrzosek, B. Gyongyosi, D.V. Ward, F. Grabherr, R.R. Gerner, A. Pfister, B. Enrich, D. Ciocan, S. Macheiner, L. Mayr, M. Drach, P. Moser, A.R. Moschen, G. Perlemuter, G. Szabo, A.M. Cassard, H. Tilg, Gut 67 (2018) 891–901.
[28]J. Li, S. Lin, P.M. Vanhoutte, C.W. Woo, A. Xu, Circulation 133 (2016) 2434–2446.
[29]W.M. de Vos, Microbiology 163 (2017) 646–648.
[30]R. Sender, S. Fuchs, R. Milo, PLoS Biol. 14 (2016) e1002533.
[31]M.G. Rooks, W.S. Garrett, Nat. Rev. Immunol. 16 (2016) 341–352.
[32]M. Nieuwdorp, P.W. Gilijamse, N. Pai, L.M. Kaplan, Gastroenterology 146 (2014) 1525–1533.
[33]M. Derrien, P. Van Baarlen, G. Hooiveld, E. Norin, M. Müller, W.M. de Vos, Front. Microbiol. 2 (2011) 166.
[34]N. Ottman, J. Reunanen, M. Meijerink, T.E. Pietilä, V. Kainulainen, J. Klievink, L. Huuskonen, S. Aalvink, M. Skurnik, S. Boeren, R. Satokari, A. Mercenier, A. Palva, H. Smidt, W.M. de Vos, C. Belzer, PLoS One 12 (2017) e0173004.
[35]J. Shin, J.-R. Noh, D.-H. Chang, Y.-H. Kim, M.H. Kim, E.S. Lee, S. Cho, B.J. Ku, M.-S. Rhee, B.-C. Kim, C.-H. Lee, B.-K. Cho, Front. Microbiol. 10 (2019) 1137.
[36]E. Cekanaviciute, B.B. Yoo, T.F. Runia, J.W. Debelius, S. Singh, C.A. Nelson, R. Kanner, Y. Bencosme, Y.K. Lee, S.L. Hauser, E. Crabtree-Hartman, I.K. Sand, M. Gacias, Y. Zhu, P. Casaccia, B.A.C. Cree, R. Knight, S.K. Mazmanian, S.E. Baranzini, Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 10713–10718.
[37]C.A. Thaiss, N. Zmora, M. Levy, E. Elinav, Nature 535 (2016) 65–74.
[38]E. Le Poul, C. Loison, S. Struyf, J.-Y. Springael, V. Lannoy, M.-E. Decobecq, S. Brezillon, V. Dupriez, G. Vassart, J. Van Damme, M. Parmentier, M. Detheux, J. Biol. Chem. 278 (2003) 25481–25489.
[39]K.M. Maslowski, A.T. Vieira, A. Ng, J. Kranich, F. Sierro, D. Yu, H.C. Schilter, M.S. Rolph, F. Mackay, D. Artis, R.J. Xavier, M.M. Teixeira, C.R. Mackay, Nature 461 (2009) 1282–1286.
[40]A. Di Lorenzo, E. Bolli, L. Tarone, F. Cavallo, L. Conti, Int. J. Mol. Sci. 21 (2020).[41]H. Plovier, A. Everard, C. Druart, C. Depommier, M. Van Hul, L. Geurts, J. Chilloux, N. Ottman, T. Duparc, L. Lichtenstein, A. Myridakis, N.M. Delzenne, J. Klievink, A. Bhattacharjee, K.C.H. van der Ark, S. Aalvink, L.O. Martinez, M.-E. Dumas, D. Maiter, A. Loumaye, M.P. Hermans, J.-P. Thissen, C. Belzer, W.M. de Vos, P.D. Cani, Nat. Med. 23 (2017) 107–113.
[42]H.S. Yoon, C.H. Cho, M.S. Yun, S.J. Jang, H.J. You, J.-H. Kim, D. Han, K.H. Cha, S.H. Moon, K. Lee, Y.-J. Kim, S.-J. Lee, T.-W. Nam, G. Ko, Nat Microbiol 6 (2021) 563–573.
[43]Z.-W. Luo, K. Xia, Y.-W. Liu, J.-H. Liu, S.-S. Rao, X.-K. Hu, C.-Y. Chen, R. Xu, Z.-X. Wang, H. Xie, Int. J. Nanomedicine 16 (2021) 2949–2963.
[44]D.R. Donohoe, N. Garge, X. Zhang, W. Sun, T.M. O’Connell, M.K. Bunger, S.J. Bultman, Cell Metab. 13 (2011) 517–526.
[45]P.V. Bauer, S.C. Hamr, F.A. Duca, Cell. Mol. Life Sci. 73 (2016) 737–755.
[46]A.L. Kau, P.P. Ahern, N.W. Griffin, A.L. Goodman, J.I. Gordon, Nature 474 (2011) 327–336.
[47]A. Schwiertz, D. Taras, K. Schäfer, S. Beijer, N.A. Bos, C. Donus, P.D. Hardt, Obesity 18 (2010) 190–195.
[48]E.S. Bliss, E. Whiteside, Front. Physiol. 9 (2018) 900.
[49]J.K. Nicholson, E. Holmes, J. Kinross, R. Burcelin, G. Gibson, W. Jia, S. Pettersson, Science 336 (2012) 1262–1267.
[50]M. Kasubuchi, S. Hasegawa, T. Hiramatsu, A. Ichimura, I. Kimura, Nutrients 7 (2015) 2839–2849.
[51]M.K. Nøhr, K.L. Egerod, S.H. Christiansen, A. Gille, S. Offermanns, T.W. Schwartz, M. Møller, Neuroscience 290 (2015) 126–137.
[52]G. Frost, M.L. Sleeth, M. Sahuri-Arisoylu, B. Lizarbe, S. Cerdan, L. Brody, J. Anastasovska, S. Ghourab, M. Hankir, S. Zhang, D. Carling, J.R. Swann, G. Gibson, A. Viardot, D. Morrison, E. Louise Thomas, J.D. Bell, Nat. Commun. 5 (2014) 3611.
[53]C.S. Byrne, E.S. Chambers, H. Alhabeeb, N. Chhina, D.J. Morrison, T. Preston, C. Tedford, J. Fitzpatrick, C. Irani, A. Busza, I. Garcia-Perez, S. Fountana, E. Holmes, A.P. Goldstone, G.S. Frost, Am. J. Clin. Nutr. 104 (2016) 5–14.
[54]H.V. Lin, A. Frassetto, E.J. Kowalik Jr, A.R. Nawrocki, M.M. Lu, J.R. Kosinski, J.A. Hubert, D. Szeto, X. Yao, G. Forrest, D.J. Marsh, PLoS One 7 (2012) e35240.
[55]J. Yan, L. Sheng, H. Li, Gut Microbes 13 (2021) 1984104.
[56]S. Kim, Y. Lee, Y. Kim, Y. Seo, H. Lee, J. Ha, J. Lee, Y. Choi, H. Oh, Y. Yoon, Appl. Environ. Microbiol. 86 (2020).
[57]C. Depommier, A. Everard, C. Druart, H. Plovier, M. Van Hul, S. Vieira-Silva, G. Falony, J. Raes, D. Maiter, N.M. Delzenne, M. de Barsy, A. Loumaye, M.P. Hermans, J.-P. Thissen, W.M. de Vos, P.D. Cani, Nat. Med. 25 (2019) 1096–1103.
[58]C. López-Otín, M.A. Blasco, L. Partridge, M. Serrano, G. Kroemer, Cell 186 (2023) 243–278.
[59]P.W. O’Toole, I.B. Jeffery, Science 350 (2015) 1214–1215.
[60]P. Pellanda, T.S. Ghosh, P.W. O’Toole, Curr. Opin. Biotechnol. 70 (2021) 48–55.
[61]H. Singh, M.G. Torralba, K.J. Moncera, L. DiLello, J. Petrini, K.E. Nelson, R. Pieper, Geroscience 41 (2019) 907–921.
[62]C. Bárcena, R. Valdés-Mas, P. Mayoral, C. Garabaya, S. Durand, F. Rodríguez, M.T. Fernández-García, N. Salazar, A.M. Nogacka, N. Garatachea, N. Bossut, F. Aprahamian, A. Lucia, G. Kroemer, J.M.P. Freije, P.M. Quirós, C. López-Otín, Nat. Med. 25 (2019) 1234–1242.
[63]V. Palmas, S. Pisanu, V. Madau, E. Casula, A. Deledda, R. Cusano, P. Uva, A. Loviselli, F. Velluzzi, A. Manzin, Nutrients 14 (2022).
[64]Z. Luan, G. Sun, Y. Huang, Y. Yang, R. Yang, C. Li, T. Wang, D. Tan, S. Qi, C. Jun, C. Wang, S. Wang, Y. Zhao, Y. Jing, Front. Microbiol. 11 (2020) 1474.
[65]J. Shin, J.-R. Noh, D. Choe, N. Lee, Y. Song, S. Cho, E.-J. Kang, M.-J. Go, S.K. Ha, D.-H. Chang, J.-H. Kim, Y.-H. Kim, K.-S. Kim, H. Jung, M.H. Kim, B.-H. Sung, S.-G. Lee, D.-H. Lee, B.-C. Kim, C.-H. Lee, B.-K. Cho, Microbiome 9 (2021) 240.
[66]E.D.-D. Cerro, M. Lambea, J. Félix, N. Salazar, M. Gueimonde, M. De la Fuente, Biogerontology 23 (2022) 35–52.
[67]J. Ma, Z. Liu, X. Gao, Y. Bao, Y. Hong, X. He, W. Zhu, Y. Li, W. Huang, N. Zheng, L. Sheng, B. Zhou, H. Chen, H. Li, Pharmacol. Res. 189 (2023) 106687.
[68]S.-Y. Zeng, Y.-F. Liu, J.-H. Liu, Z.-L. Zeng, H. Xie, J.-H. Liu, Aging Dis. 14 (2023) 2015–2027.
[69]J. Lee, V.R. Venna, D.J. Durgan, H. Shi, J. Hudobenko, N. Putluri, J. Petrosino, L.D. McCullough, R.M. Bryan, Gut Microbes 12 (2020) 1–14.
[70]S. Sanna, N.R. van Zuydam, A. Mahajan, A. Kurilshikov, A. Vich Vila, U. Võsa, Z. Mujagic, A.A.M. Masclee, D.M.A.E. Jonkers, M. Oosting, L.A.B. Joosten, M.G. Netea, L. Franke, A. Zhernakova, J. Fu, C. Wijmenga, M.I. McCarthy, Nat. Genet. 51 (2019) 600–605.
[71]M. Müller, M.A.G. Hernández, G.H. Goossens, D. Reijnders, J.J. Holst, J.W.E. Jocken, H. van Eijk, E.E. Canfora, E.E. Blaak, Sci. Rep. 9 (2019) 12515.|
[72]J.L. Pluznick, R.J. Protzko, H. Gevorgyan, Z. Peterlin, A. Sipos, J. Han, I. Brunet, L.-X. Wan, F. Rey, T. Wang, S.J. Firestein, M. Yanagisawa, J.I. Gordon, A. Eichmann, J. Peti-Peterdi, M.J. Caplan, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 4410–4415.
[73]S. Sharma, R. Taliyan, S. Singh, Behav. Brain Res. 291 (2015) 306–314.
[74]N. Govindarajan, R.C. Agis-Balboa, J. Walter, F. Sananbenesi, A. Fischer, J. Alzheimers. Dis. 26 (2011) 187–197.
[75]N. Ottman, S.Y. Geerlings, S. Aalvink, W.M. de Vos, C. Belzer, Best Pract. Res. Clin. Gastroenterol. 31 (2017) 637–642.
[76]M. Zheng, R. Han, Y. Yuan, Y. Xing, W. Zhang, Z. Sun, Y. Liu, J. Li, T. Mao, Front. Immunol. 13 (2022) 1089600.
[77]K. Zhou, J. Funct. Foods 33 (2017) 194–201.
[78]G.R. Gibson, R. Hutkins, M.E. Sanders, S.L. Prescott, R.A. Reimer, S.J. Salminen, K. Scott, C. Stanton, K.S. Swanson, P.D. Cani, K. Verbeke, G. Reid, Nat. Rev. Gastroenterol. Hepatol. 14 (2017) 491–502.
[79]A. Everard, V. Lazarevic, M. Derrien, M. Girard, G.G. Muccioli, A.M. Neyrinck, S. Possemiers, A. Van Holle, P. François, W.M. de Vos, N.M. Delzenne, J. Schrenzel, P.D. Cani, Diabetes 60 (2011) 2775–2786.
[80]N. Roshanravan, R. Mahdavi, E. Alizadeh, A. Ghavami, Y. Rahbar Saadat, N. Mesri Alamdari, S. Alipour, M.R. Dastouri, A. Ostadrahimi, J Cardiovasc Thorac Res 9 (2017) 183–190.
[81]J.R. Bush, J. Baisley, S.V. Harding, M.J. Alfa, Nutrients 15 (2023).
[82]S. Mithul Aravind, S. Wichienchot, R. Tsao, S. Ramakrishnan, S. Chakkaravarthi, Food Res. Int. 142 (2021) 110189.
[83]F.F. Anhê, G. Pilon, D. Roy, Y. Desjardins, E. Levy, A. Marette, Gut Microbes 7 (2016) 146–153.
[84]M.C. Rodríguez-Daza, W.M. de Vos, Int. J. Mol. Sci. 24 (2022).
[85]A. González-Sarrías, M. Romo-Vaquero, R. García-Villalba, A. Cortés-Martín, M.V. Selma, J.C. Espín, Mol. Nutr. Food Res. 62 (2018) e1800160.
[86]Z. Li, S.M. Henning, R.-P. Lee, Q.-Y. Lu, P.H. Summanen, G. Thames, K. Corbett, J. Downes, C.-H. Tseng, S.M. Finegold, D. Heber, Food Funct. 6 (2015) 2487–2495.
[87]R. Zhao, X. Long, J. Yang, L. Du, X. Zhang, J. Li, C. Hou, Food Funct. 10 (2019) 8273–8285.
[88]A.V. Ropot, A.M. Karamzin, O.V. Sergeyev, Curr. Microbiol. 77 (2020) 1363–1372.
[89]L. Lavefve, L.R. Howard, F. Carbonero, Food Funct. 11 (2020) 45–65.
[90]H.W. Jeong, J.K. Kim, A.Y. Kim, D. Cho, J.-H. Lee, J.K. Choi, M. Park, W. Kim, J. Med. Food 23 (2020) 841–851.

No Comments Yet
Sign in or Register to Comment