Introduction to Dopamine
Dopamine is one of the main neurotransmitters in the brain. It is most commonly recognized for its role in reward, motivation, and pleasure, but also plays a crucial part in modulating focus, motivation, cognitive flexibility, and emotional resilience. In addition to these creative-productive capacities and states, dopamine is one of the main regulators of motor control and coordination of body movements.[1–5]
Dopaminergic substances or actions affect dopamine-related activity in the brain. The proper functioning of the dopaminergic system is of high importance for cognitive performance and emotional drive. This article reviews the dopaminergic system, including what dopamine is, what dopamine precursors are, where and how it is metabolized, and how the system can be supported.
What Is Dopamine?
Dopamine is one of the three main signaling molecules from the catecholamine family. The other two are the famous (or perhaps infamous) fight-or-flight response molecules epinephrine (adrenaline) and norepinephrine (noradrenaline).[6]
Dopamine is produced in the brain. It’s also made in and used by other systems in the body, where it acts as an important chemical messenger. Dopamine is involved with the heart, modulating cardiovascular function, stimulating heart muscle contraction, and promoting the widening of blood vessels needed for proper blood flow. Dopamine is used in the kidneys to help them function properly, stimulating increased urination, and excreting excess sodium (salt). Dopamine also modulates the immune system and the activity of lymphocytes (a type of white blood cell that plays a vital role in the immune system’s ability to protect us).[7]
Dopamine neurons are relatively few in number—only 1% of brain neurons are dopaminergic—but have a big impact on creative-productive capacities, emotions, and coordination of body movements.
The brain needs to let certain things in (like nutrients) and out (such as metabolic waste products), and protect itself against the entry of other things (like bacteria). It does this with the blood-brain barrier, which acts a bit like a doorman, choosing what does and doesn’t gain access. Dopamine does not cross the blood-brain barrier and therefore all brain dopamine has to be produced locally in the dopaminergic nerve cells (neurons) in the brain from dopamine-building block molecules.[8]
While dopamine promotes many critical brain capacities and states, only about 1% of brain neurons are dopaminergic. These relatively few neurons must be protected and supported if we want to achieve and sustain peak brain performance throughout our lifespan. We must have adequate dopamine levels to function properly.
Where Is Dopamine Created in the Brain? Where Is It Used? What Does It Do?
Dopaminergic neurons are confined to a few small—in size but not importance—brain areas. But this doesn’t mean dopamine’s brain actions are confined to these regions alone. Dopaminergic neurons have nerve fibers (axons) that connect them to other neurons. These nerve fibers are used to transmit dopamine-related information to neurons in other parts of the brain. It’s through these axons that dopamine exerts its modulatory effects elsewhere in the brain.[1]
The major dopamine-producing region of the brain is the midbrain, which contains the vast majority of dopaminergic neurons. Within the midbrain, the substantia nigra is the most prominent dopaminergic cluster of neurons and has an important function in the control of movement and reflexes.
The ventral tegmental area (VTA) is another important dopaminergic area in the midbrain. Its projections to the nucleus accumbens, olfactory tubercle, amygdala, and hippocampus make up another of the major brain dopamine pathways—the mesolimbic dopaminergic pathway. This pathway, also known as the reward pathway, plays a major role in reward, in the motivational component of reward-motivated behavior, in behavioral reinforcement, and in the perception of pleasure.[1–3]
Dopaminergic pathways in the brain influence (1) reward processes (i.e., wanting and liking, and the reinforcement of pleasure behaviors), (2) executive functions (i.e., goal-directed behaviors, cognitive flexibility, and problem solving), (3) associative learning (i.e., acquiring and modifying behaviors, skills, etc.), and (4) motor control (i.e., coordination and control of reflexes and voluntary movements).
Projections from the VTA to the brain regions called the prefrontal cortex and cingulate cortex constitute a third major brain dopamine pathway called the mesocortical dopaminergic pathway, which is involved in cognitive control, behavioral flexibility, and emotional resilience. Through this pathway, dopamine plays a significant role in modulating executive function, the set of higher-order cognitive processes that underlie goal-directed behaviors of impulse control, response inhibition, attention, working memory, cognitive flexibility, planning, judgment, and decision-making.[5,9] Dopaminergic activity in the medial prefrontal cortex mediates important aspects of executive function, particularly cognitive flexibility, set-shifting (task-switching), and attention. Higher levels of dopamine are associated with optimal performance of these processes.[1,4,5]
Neurotransmitters—dopamine, serotonin, glutamate, GABA, acetylcholine, etc—are chemical messengers that convey information from one neuron (message sender) to another "target" neuron (message recipient). The small space between the two is called a synapse [Note: An adult human brain is estimated to contain 100–500 trillion synapses!]
Dopamine message-receiving neurons have receptors—think of them as being akin to the neuron’s eyes or ears—that listen/watch for a dopamine message. When the receptors detect dopamine, they are activated, which leads to excitation (i.e., turning on) or inhibition (i.e., turning off), depending on the specific types of dopamine receptors on the target neuron.
While this might seem complicated, the key things to remember are (1) the response to dopamine of any individual neuron will depend on the types of receptors on the target neurons and the amount of dopamine released by the sending neurons, (2) the dopamine message (i.e., the release of dopamine and activation of the receptor) will be brief, but the effects it has will last much longer, and (3) dopamine has big effects (despite being made in only small areas of the brain) because the dopamine pathways connect to areas of the brain affecting voluntary and involuntary muscle movements, emotional life, and creative-productive capacities and states.
Dopamine Synthesis, Signaling, and Cleanup
Neurotransmitters have several characteristics in common. The first is that they are synthesized (i.e., made or created) in neurons. After that, they are moved into areas near the end of neurons (synaptic vesicles) where they are stored until needed. This occurs in preparation for signaling, which involves the release of the neurotransmitter from the message-sending neuron into the space between neurons (synaptic cleft), so it can activate (i.e., bind to) receptors on message-receiving neurons. After this signal is sent, the space between neurons needs to be cleaned up, so it can be made ready for the next time a message needs to be sent. This is achieved by both re-absorbing the neurotransmitter so it can be reused (recycling), or by degrading (breaking down) the neurotransmitter. Let’s explore how these occur with dopamine.
"A rate-limiting step is the slowest step in a metabolic pathway. It determines the overall rate that the ultimate product will be produced. Traffic is often used as an analogy, where the rate-limiting step would be the place where traffic gets congested."
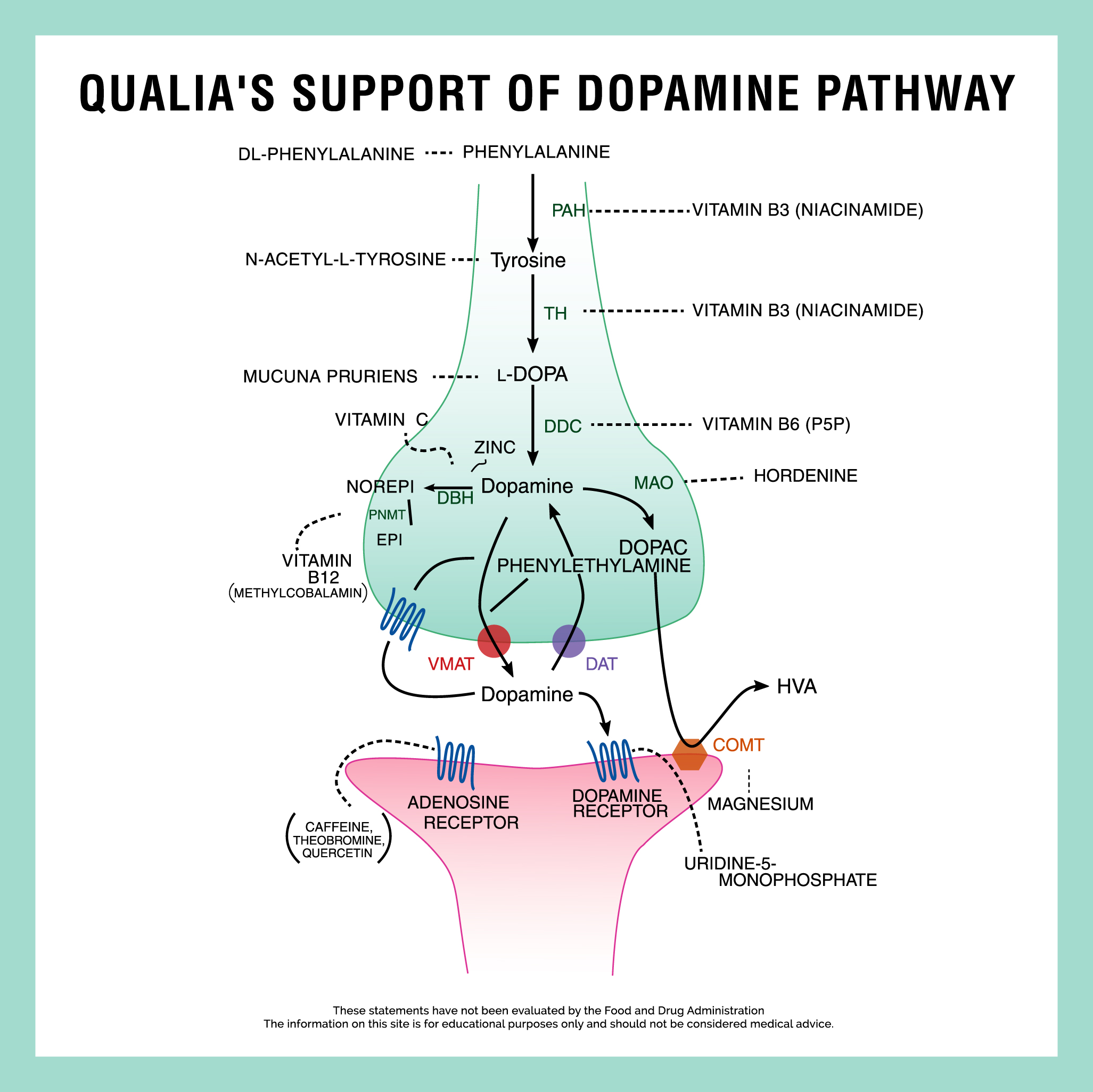
Let’s look at how this works if we start with the L-tyrosine building block. Dopamine is synthesized both at the nerve terminals, and in the cell bodies of dopaminergic neurons, from the amino acid L-tyrosine (which as mentioned can be made from L-phenylalanine). The enzyme tyrosine hydroxylase (TH) converts L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), which in turn is converted to dopamine by aromatic-L-amino-acid decarboxylase (AAAD, also known as DOPA decarboxylase [DDC]) with pyridoxal-5’-phosphate (the active form of vitamin B6) as the coenzyme.[6] [Note: Enzymes are catalysts used to produce specific biochemical reactions: They usually have names that end in “ase.” Coenzymes are parts of certain enzymes. Many coenzymes are derived from vitamins.]
The synthesis of L-DOPA by tyrosine hydroxylase (TH) is considered the rate-limiting step (i.e., the slowest step in the pathway, so akin to a bottleneck, which is the most likely place for a metabolic traffic jam to occur) in the synthesis of dopamine. Since dopamine itself is the precursor to the synthesis of the other catecholamine neurotransmitters, norepinephrine and epinephrine (in noradrenergic and adrenergic neurons, respectively), tyrosine hydroxylase (TH) functionally is the limiting enzyme in the synthesis of the three catecholamine neurotransmitters.[6,10]
After synthesis, dopamine is transported into synaptic vesicles by a vesicular monoamine transporter. Dopamine is stored in these vesicles until an action potential, through the influx of calcium, triggers its release into the synaptic cleft. This transport and storage occur in the message-sending neuron in anticipation of needing to send dopamine messages in the future. The calcium influx is the biological cue to send the message, so it starts the signaling process.
Once released, dopamine binds to and activates either postsynaptic (i.e., message-receiving) receptors or presynaptic (i.e., message-sending) autoreceptors. When an action potential is elicited in the postsynaptic neuron, dopamine molecules are released from their receptors and retaken up into the presynaptic cell or into surrounding glial cells by the dopamine transporter (DAT) or by the plasma membrane monoamine transporter (VMAT). This is the recycling part of the cleanup process. Once back in the cell, dopamine is either broken down or repackaged into vesicles for future reuse.[6]
There are complex regulatory systems in place to make sure that neurotransmitters aren’t left floating around. So, cleanup is an essential process for efficient signaling. Cleanup is partly because dopamine’s too important a resource to waste. It’s also partly because of how signaling works.
Signaling is based on change. It’s not the amount of something that produces a response; it’s the change in the amount. Light serves as a useful analogy. If you were to light a single candle in a dark room you’d notice the difference. If you lit one additional candle in a room with another hundred lit candles, you might not. Neurotransmitters work on this relative change principle. Short bursts of dopamine release produce responses. But, for the best response to occur with the smallest amount of dopamine, the space between neurons needs to be the equivalent of a dark room, not already brightly lit by dopamine candles.
The dopamine cleanup pathways rely on multiple enzymes working in concert for recycling and degradation. These will be briefly described below. The important thing to understand is that support of dopamine signaling also means supporting these enzymes (as a reminder, enzymes have names that end in “ase”).
Within the cell, dopamine is degraded into inactive metabolites by monoamine oxidase (MAO). MAO catalyzes the oxidative deamination of dopamine into a metabolite called DOPAL, which is then converted into DOPAC by the aldehyde dehydrogenase (ALDH) and then into homovanillic acid (HVA), the primary metabolite of dopamine, by catechol-O-methyltransferase (COMT). There are two isozymes of MAO, named MAO-A and MAO-B, located on the outer membrane of mitochondria. Both isoforms can metabolize dopamine. MAO-A is most abundant in catecholaminergic neurons, whereas MAO-B is predominantly found in glial cells (and serotonergic and histaminergic neurons).[6,11] [Note: Glial cells (or neuroglia or simply glia) are non-neuronal brain cells that provide support and protection for neurons.]
Dopamine can also be metabolized (i.e., inactivated) by COMT into 3-methoxytyramine, using SAMe as a methyl donor, and then converted into HVA by MAO and ALDH. COMT is synthesized in two forms, soluble (i.e., free-floating) and membrane-bound; the latter is the primary form in the brain. COMT is dependent on magnesium and inhibited by calcium. COMT can also metabolize L-DOPA.[6,12] COMT is predominantly found extracellularly (i.e., in the space outside neurons) bound to the outer membrane of postsynaptic (i.e., message receiving) neurons, but low levels may also exist in glia.
In the brain, COMT-dependent extracellular dopamine inactivation is of particular importance in brain regions with low expression of the presynaptic dopamine transporter—the recycling mechanism that takes up and returns dopamine into the message-sending neurons for inactivation or reuse. When the message-sending neurons are not designed to take up and recycle dopamine, it’s critical to have this degradation backup plan to inactivate it, keeping the space between neurons the equivalent of a dark room for the next time a dopamine burst is sent.
Dopamine Signaling Stack
Key parts of designing a dopamine stack are: (1) augment the precursor pool of compounds used to make it; (2) give full pathway support; (3) support enzyme function involved in dopamine synthesis, signaling, and cleanup; and (4) promote balanced signaling and neuroprotection. Let’s put these pieces together now.
The rate-limiting step in this L-DOPA pathway is the enzyme tyrosine hydroxylase. This is the enzymatic step that turns L-tyrosine into L-DOPA. Mucuna pruriens—a member of the legume (i.e., bean) family—is included in this stack because it is a natural source of L-DOPA*[13] which can enter the pathway after this step. We use this herbal ingredient to supply an amount of L-DOPA that a person would consume if they ate about 3-6 ounces of fava beans (fava beans are considered one of the richest food sources of L-DOPA). This ingredient thereby supports the production of dopamine and bypasses the potential metabolic traffic jam.
While L-DOPA is further along the pathway than L-tyrosine, there’s one more enzymatic step to go to get to dopamine. Vitamin B6, as Pyridoxal 5’-phosphate (P5P), is included in the stack to support the conversion of L-DOPA to dopamine.* The enzyme DOPA decarboxylase (DDC) is responsible for converting L-DOPA to dopamine and requires P5P as the coenzyme form of vitamin B6 to exert its function.[14]
With the goal of full pathway support in mind, two amino acid dopamine building blocks are included. As mentioned, L-tyrosine is a conditionally essential amino acid and the direct precursor to L-DOPA. It is included in this stack in the form of N-Acetyl-L-Tyrosine to augment the supply of L-tyrosine available for L-DOPA (and then dopamine) synthesis. L-tyrosine is synthesized from the essential amino acid L-phenylalanine by the enzyme phenylalanine hydroxylase.[6] We include DL-Phenylalanine in the stack to support healthy levels of L-phenylalanine available for L-tyrosine synthesis, furthering our full pathway support goal.
By including Mucuna pruriens, N-Acetyl-L-Tyrosine, and DL-Phenylalanine, the stack supports three different steps, with different kinetics, of the dopamine synthesis pathway, allowing for a prolonged and sustained availability of precursor resources to be recruited for its synthesis.*
Deficiency in any precursor amino acid or any cofactor in the catecholaminergic anabolic pathways can impair the synthesis of all three catecholamine neurotransmitters. Both tyrosine hydroxylase (i.e., TH is the enzyme that makes L-DOPA from L-tyrosine) and phenylalanine hydroxylase (i.e., PAH is the enzyme that makes L-tyrosine from L-phenylalanine) require tetrahydrobiopterin as a coenzyme. Tetrahydrobiopterin is synthesized from guanosine triphosphate (GTP) through an NADPH-dependent pathway.[15] Vitamin B3, as Niacinamide, is a precursor to NADPH,[16] and can therefore indirectly support the activity of both enzymes in the pathway which starts with building block molecules and finishes with dopamine. Vitamin C is a cofactor in the conversion of dopamine to norepinephrine by dopamine beta-hydroxylase (DBH).
Designing a neurotransmitter stack includes supplying building blocks used in a pathway and supporting the enzyme reactions that build new molecules. It also includes supporting the signaling processes involved in listening for and responding to neurotransmitter messages.
Uridine Monophosphate has a unique role in the dopamine stack. Although uridine may decrease the density of dopamine receptors, it seems to enhance their signal transduction and turnover rate, leading to an increase in dopamine-dependent behaviors.[17] In other words, it may promote improved results on the message-receiving side (listening end) of the dopamine signaling process.* Uridine Monophosphate also enhances potassium-evoked dopamine release.*[18]
Caffeine and Theobromine play a role in this stack due to their antagonism (i.e., slowing the activation) of adenosine receptors.* Since adenosine receptor activation decreases dopaminergic activity, slowing activity of adenosine receptors can indirectly contribute to enhanced dopaminergic signaling.[19–22]
Why Should You Support Dopaminergic Pathways and Processes?
Dopamine modulates a wide array of cognitive capacities and emotional states. But what happens if demands for dopamine signaling exceed supplies? Or if there are obstacles interfering with or slowing dopamine creation, signaling, or cleanup processes?
The goal is not to try to control the dopamine system. Instead, using a complexity science approach, the goal is to provide resources, support regulatory functions and dopamine levels at multiple points along the interconnected pathways (especially points where there’s likely to be a metabolic traffic jam), and allow the complex adaptive system to determine how to allocate the resources to meet the emergent needs of the different processes involved in accomplishing its objectives.
When we formulate a dopamine stack, it’s designed to supplement the brain’s building blocks of the precursor nutrients needed for dopamine-related creative-productive capacities and states. In addition to these resources, it’s also designed to provide support for the reactions that are required for the optimal performance of dopaminergic pathways and processes. It’s important to boost dopamine to proper levels to reach your full potential. Increased dopamine can be very beneficial to your reward system. Dopamine plays a prominent role in your physical and mental health. It’s also responsible for your reward system, improved mood, feelings of pleasure and more. Listening to music and exercise can also increase low dopamine levels in the brain.
Why supply these resources? Why support the dopamine signaling pathways and processes? The big picture answer is that these dopamine pathways and processes play a critical part in boosting motivation, focus, and cognitive flexibility, while also promoting a healthy emotional life, including sensations of reward and pleasure. Boosting dopamine can be very beneficial for you. The ultimate complexity science answer is a response, which in this case is about the optimization of creative and productive flow states.*
References
[1]S.J. Chinta, J.K. Andersen, Int. J. Biochem. Cell Biol. 37 (2005) 942–946.
[2]E.S. Bromberg-Martin, M. Matsumoto, O. Hikosaka, Neuron 68 (2010) 815–834.
[3]K.C. Berridge, M.L. Kringelbach, Neuron 86 (2015) 646–664.
[4]T. Goschke, A. Bolte, Neuropsychologia 62 (2014) 403–423.
[5]S.F. Logue, T.J. Gould, Pharmacol. Biochem. Behav. 123 (2014) 45–54.
[6]M.E. Gnegy, in: S.T. Brady, G.J. Siegel, R.W. Albers, D.L. Price (Eds.), Basic Neurochemistry (Eighth Edition), Academic Press, New York, 2012, pp. 283–299.
[7]F. Amenta, A. Ricci, S.K. Tayebati, D. Zaccheo, Ital. J. Anat. Embryol. 107 (2002) 145–167.
[8]Purves, Neuroscience, 5th edition, Sinauer Associates, 2011.
[9]D.T. Stuss, M.P. Alexander, Psychol. Res. 63 (2000) 289–298.
[10]S.C. Daubner, T. Le, S. Wang, Arch. Biochem. Biophys. 508 (2011) 1–12.
[11]J.C. Shih, K. Chen, M.J. Ridd, Annu. Rev. Neurosci. 22 (1999) 197–217.
[12]E. Nissinen, P.T. Männistö, Int. Rev. Neurobiol. 95 (2010) 73–118.
[13]H. Pulikkalpura, R. Kurup, P.J. Mathew, S. Baby, Sci. Rep. 5 (2015) 11078.
[14]R. Montioli, B. Cellini, M. Dindo, E. Oppici, C.B. Voltattorni, Biomed Res. Int. 2013 (2013) 161456.
[15]B. Thöny, G. Auerbach, N. Blau, Biochem. J 347 Pt 1 (2000) 1–16.
[16]P. Belenky, K.L. Bogan, C. Brenner, Trends Biochem. Sci. 32 (2007) 12–19.
[17]C. Farabegoli, E. Merlo Pich, M. Cimino, L.F. Agnati, K. Fuxe, Acta Physiol. Scand. 132 (1988) 209–216.
[18]L. Wang, A.M. Pooler, M.A. Albrecht, R.J. Wurtman, J. Mol. Neurosci. 27 (2005) 137–145.
[19]B.B. Fredholm, Pharmacol. Toxicol. 76 (1995) 93–101.
[20]G. Burnstock, Advances in Experimental Medicine and Biology 986 (2013) 1–12.
[21]R. Franco, A. Oñatibia-Astibia, E. Martínez-Pinilla, Nutrients 5 (2013) 4159–4173.
[22]C.A. Olson, J.A. Thornton, G.E. Adam, H.R. Lieberman, J. Clin. Psychopharmacol. 30 (2010) 573–578.







Christina Efstathopoulos