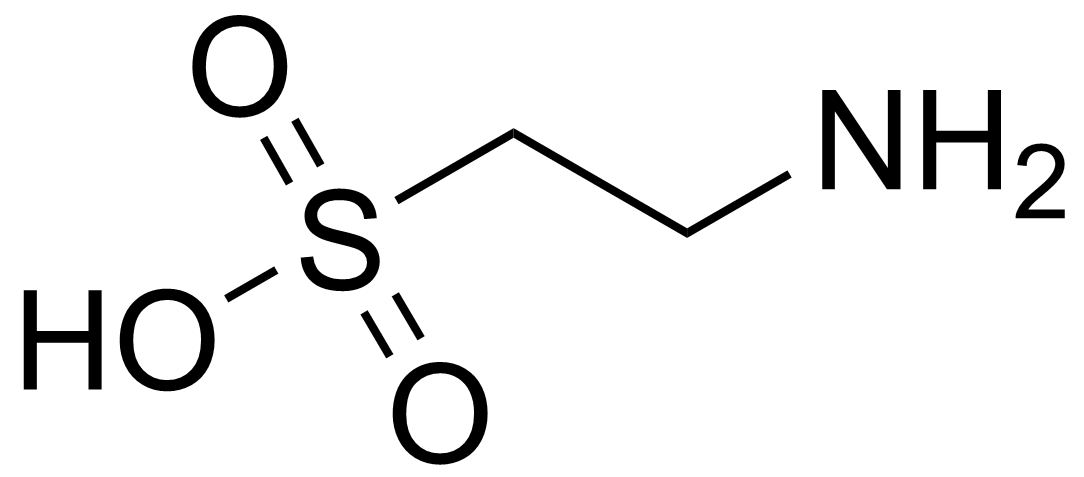
Taurine
COMMON NAME
Taurine
Top Benefits of Taurine
Supports brain health *
Supports healthy vision *
Supports vascular health *
What is Taurine?
Taurine (2-aminoethanesulfonic acid) is a sulfur-containing amino acid. It is found in the diet and can be produced in the human body, with the combination determining body stores. Animal products, including shellfish, fish, poultry and red meat, are the best food source. Vegan diets contain almost no taurine [1]. Taurine is present in nearly all tissues, and is the most abundant free amino acid in muscle, heart, brain, and retina. Taurine has important roles in the human body in osmoregulation, as an antioxidant, and as a neuromodulator. Taurine is present in all ocular tissues—retina, lens, cornea, etc.—and is critical for retinal and photoreceptor cell function. Taurine also supports neuroprotective functions in the central nervous system [2,3]. Taurine may facilitate the bioavailability of lipid nutrients [4].*
Neurohacker’s Taurine Sourcing
Taurine is non-GMO and vegan.
Taurine Formulating Principles and Rationale
Dietary intake of taurine in omnivores has been estimated to be in the range of 75-135 mg a day, while subsets with the highest intakes may average 200-500 mg. Vegan diets are virtually devoid of taurine [5,6] and vegans have lower blood levels of it [1]. Taurine has been used in clinical studies at amounts ranging from a few hundred milligrams to a few grams. When used in combination with other ingredients, taurine has been used at lower amounts. Neurohacker believes taurine follows a threshold response (see Neurohacker Dosing Principles) when given to healthy people, which means the majority of functional benefits occur within the range of average-to-high dietary intake. The amount of taurine included in a Neurohacker Collective product can vary depending on the product’s goal; however, we generally dose taurine closer to, but still above, the average dietary intake. The goal is to provide a supplemental serving of taurine that ensures adequate intake even for vegans and vegetarians.*
Taurine Key Mechanisms
Supports brain function*
Supports synaptic long-term potentiation* [7]
Supports GABAergic neurotransmission* [8–11]
Supports glycinergic neurotransmission* [12]
Supports brain-derived neurotrophic factor (BDNF)* [11]
Supports neuroprotective functions* [13]
Supports cerebral blood flow* [13]
Supports neuronal mitochondrial function* [13]
Supports positive affective responses and calm behaviors (in animals)* [12,14–18]
Supports vision*
Supports resistance to visual fatigue* [19]
Supports synaptic transmission in retinal ganglion cells* [20]
Supports eyes against stress from blue light and ultraviolet light* [21–23]
Supports retinal and optic nerve neuroprotective functions* [24–33]
Supports retinal antioxidant defense functions* [26,27,34]
Supports photoreceptor cell visual function* [24]
Supports mitochondrial function and antioxidant defenses*
Supports mitochondrial respiratory chain function* [35,36]
Supports antioxidant defenses* [37–40]
Supports tissue protection from oxidative damage* [15,40–42]
Supports healthy cardiovascular function*
Supports healthy vascular endothelial cell function* [43–45]
Supports healthy cardiac muscle cell function* [41,42]
Supports healthy blood flow* [43,45]
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
REFERENCES
[1]S.A. Laidlaw, T.D. Shultz, J.T. Cecchino, J.D. Kopple, Am. J. Clin. Nutr. 47 (1988) 660–663.
[2]S.S. Oja, P. Saransaari, Adv. Exp. Med. Biol. 975 Pt 1 (2017) 89–94.
[3]H. Ripps, W. Shen, Mol. Vis. 18 (2012) 2673–2686.
[4]A.M. Petrosian, J.E. Haroutounian, Amino Acids 19 (2000) 409–421.
[5]S.K. Rana, T.A. Sanders, Br. J. Nutr. 56 (1986) 17–27.
[6]S.A. Laidlaw, M. Grosvenor, J.D. Kopple, JPEN J. Parenter. Enteral Nutr. 14 (1990) 183–188.
[7]N. del Olmo, L.M. Suárez, L.M. Orensanz, F. Suárez, J. Bustamante, J.M. Duarte, R. Martín del Río, J.M. Solís, Eur. J. Neurosci. 19 (2004) 1875–1886.
[8]K. Kuriyama, T. Hashimoto, in: S. Schaffer, J.B. Lombardini, R.J. Huxtable (Eds.), Taurine 3: Cellular and Regulatory Mechanisms, Springer US, Boston, MA, 1998, pp. 329–337.
[9]M.H. Bureau, R.W. Olsen, Eur. J. Pharmacol. 207 (1991) 9–16.
[10]P. Kontro, S.S. Oja, Neuropharmacology 29 (1990) 243–247.
[11]G. Caletti, F.B. Almeida, G. Agnes, M.S. Nin, H.M.T. Barros, R. Gomez, Behav. Brain Res. 283 (2015) 11–15.
[12]C.G. Zhang, S.-J. Kim, Ann. Nutr. Metab. 51 (2007) 379–386.
[13]Q. Wang, W. Fan, Y. Cai, Q. Wu, L. Mo, Z. Huang, H. Huang, Amino Acids 48 (2016) 2169–2177.
[14]W. Iio, N. Matsukawa, T. Tsukahara, A. Toyoda, Amino Acids 43 (2012) 2037–2046.
[15]G. Caletti, D.B. Olguins, E.F. Pedrollo, H.M.T. Barros, R. Gomez, Amino Acids 43 (2012) 1525–1533.
[16]A. Toyoda, W. Iio, Adv. Exp. Med. Biol. 775 (2013) 29–43.
[17]S.W. Chen, W.X. Kong, Y.J. Zhang, Y.L. Li, X.J. Mi, X.S. Mu, Life Sci. 75 (2004) 1503–1511.
[18]W.X. Kong, S.W. Chen, Y.L. Li, Y.J. Zhang, R. Wang, L. Min, X. Mi, Pharmacol. Biochem. Behav. 83 (2006) 271–276.
[19]M. Zhang, L.F. Bi, Y.D. Ai, L.P. Yang, H.B. Wang, Z.Y. Liu, M. Sekine, S. Kagamimori, Amino Acids 26 (2004) 59–63.
[20]Z. Jiang, S. Bulley, J. Guzzone, H. Ripps, W. Shen, Adv. Exp. Med. Biol. 775 (2013) 53–68.
[21]H. Pasantes-Morales, C. Cruz, Brain Res. 330 (1985) 154–157.
[22]W. Dayang, P. Dongbo, Cutan. Ocul. Toxicol. 37 (2018) 240–244.
[23]W. Dayang, P. Dongbo, Cutan. Ocul. Toxicol. 37 (2018) 90–95.
[24]Y. Tao, M. He, Q. Yang, Z. Ma, Y. Qu, W. Chen, G. Peng, D. Teng, Drug Des. Devel. Ther. 13 (2019) 2689–2702.
[25]Y. Fan, J. Lai, Y. Yuan, L. Wang, Q. Wang, F. Yuan, Curr. Eye Res. 45 (2020) 52–63.
[26]A.J.A. Jafri, R. Agarwal, I. Iezhitsa, P. Agarwal, N.M. Ismail, Amino Acids 51 (2019) 641–646.
[27]N.N. Nor Arfuzir, R. Agarwal, I. Iezhitsa, P. Agarwal, S. Sidek, N.M. Ismail, Neural Regeneration Res. 13 (2018) 2014–2021.
[28]N. Froger, F. Jammoul, D. Gaucher, L. Cadetti, H. Lorach, J. Degardin, D. Pain, E. Dubus, V. Forster, I. Ivkovic, M. Simonutti, J.-A. Sahel, S. Picaud, Adv. Exp. Med. Biol. 775 (2013) 69–83.
[29]N. Froger, L. Cadetti, H. Lorach, J. Martins, A.-P. Bemelmans, E. Dubus, J. Degardin, D. Pain, V. Forster, L. Chicaud, I. Ivkovic, M. Simonutti, S. Fouquet, F. Jammoul, T. Léveillard, R. Benosman, J.-A. Sahel, S. Picaud, PLoS One 7 (2012) e42017.
[30]K. Zeng, H. Xu, M. Mi, K. Chen, J. Zhu, L. Yi, T. Zhang, Q. Zhang, X. Yu, Neurochem. Res. 35 (2010) 1566–1574.
[31]X. Yu, Z. Xu, M. Mi, H. Xu, J. Zhu, N. Wei, K. Chen, Q. Zhang, K. Zeng, J. Wang, F. Chen, Y. Tang, Neurochem. Res. 33 (2008) 500–507.
[32]X. Yu, K. Chen, N. Wei, Q. Zhang, J. Liu, M. Mi, Br. J. Nutr. 98 (2007) 711–719.
[33]W. Hadj-Saïd, V. Fradot, I. Ivkovic, J.-A. Sahel, S. Picaud, N. Froger, Adv. Exp. Med. Biol. 975 Pt 2 (2017) 687–701.
[34]M.A.S. Di Leo, S.A. Santini, S. Cercone, D. Lepore, N. Gentiloni Silveri, S. Caputo, A.V. Greco, B. Giardina, F. Franconi, G. Ghirlanda, Amino Acids 23 (2002) 401–406.
[35]C.J. Jong, J. Azuma, S. Schaffer, Amino Acids 42 (2012) 2223–2232.
[36]S.W. Schaffer, J. Azuma, M. Mozaffari, Can. J. Physiol. Pharmacol. 87 (2009) 91–99.
[37]A.T.A. Nandhini, V. Thirunavukkarasu, M.K. Ravichandran, C.V. Anuradha, Singapore Med. J. 46 (2005) 82–87.
[38]P.S. Devamanoharan, A.H. Ali, S.D. Varma, Free Radic. Res. 29 (1998) 189–195.
[39]G. Guz, E. Oz, N. Lortlar, N.N. Ulusu, N. Nurlu, B. Demirogullari, S. Omeroglu, S. Sert, C. Karasu, Amino Acids 32 (2007) 405–411.
[40]H. Tabassum, S. Parvez, H. Rehman, B. Dev Banerjee, D. Siemen, S. Raisuddin, Hum. Exp. Toxicol. 26 (2007) 509–518.
[41]J. Hanna, R. Chahine, G. Aftimos, M. Nader, A. Mounayar, F. Esseily, S. Chamat, Exp. Toxicol. Pathol. 56 (2004) 189–194.
[42]R. Kingston, C.J. Kelly, P. Murray, Curr. Pharm. Des. 10 (2004) 2401–2410.
[43]M.A. Moloney, R.G. Casey, D.H. O’Donnell, P. Fitzgerald, C. Thompson, D.J. Bouchier-Hayes, Diab. Vasc. Dis. Res. 7 (2010) 300–310.
[44]S.-G. Ra, Y. Choi, N. Akazawa, K. Kawanaka, H. Ohmori, S. Maeda, Adv. Exp. Med. Biol. 1155 (2019) 407–414.
[45]Q. Sun, B. Wang, Y. Li, F. Sun, P. Li, W. Xia, X. Zhou, Q. Li, X. Wang, J. Chen, X. Zeng, Z. Zhao, H. He, D. Liu, Z. Zhu, Hypertension 67 (2016) 541–549.